
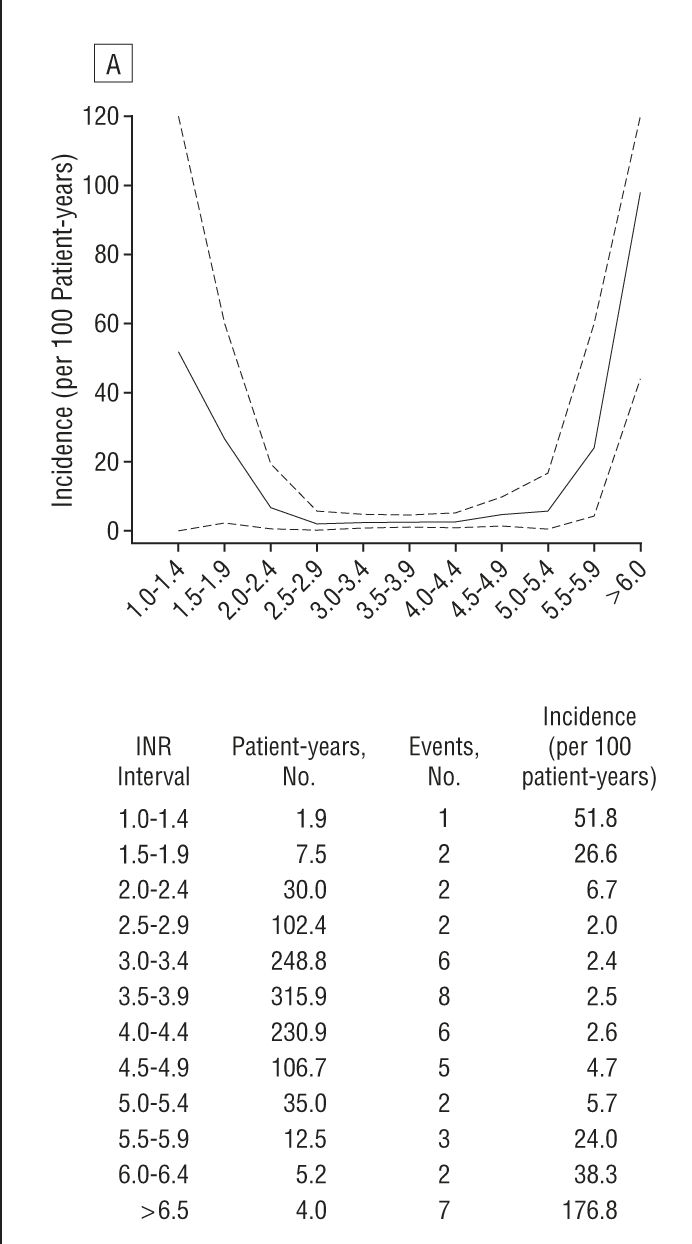
With mechanical aortic valve, VKA therapy with a target INR of 2.5 (range 2.0 to 3.0) is recommended (Grade 1B recommendation).VKA therapy is recommended over no VKA therapy for long-term management (Grade 1B recommendation).Table 1: ACCP Recommendations for Antithrombotic Therapy in Patients With Mechanical Heart Valves 12 14 The risk of major bleeding in patients with mechanical valves treated with VKA therapy was found to be 1.2 to 2.6 events per 100 patient-years. 14 VKA therapy was also superior to aspirin therapy alone for total thromboembolism risk (1.8 events per 100 patient-years 95% confidence interval, 1.7 to 1.9 versus 7.5 events per 100 patient-years 95% CI, 5.9 to 9.4) and risk of valve thrombosis (0.2 events per 100 patient-years 95% CI, 0.2 to 0.2 versus 1.0 events per 100 patient-years 95% CI, 0.4 to 1.7). When compared with no antithrombotic therapy in a 1994 meta-analysis, VKA therapy showed statistically significant reductions in the rate of major systemic embolization (from 4.0 to 1.0 events per 100 patient-years), total thromboembolism risk (from 8.6 to 1.8 events per 100 patient-years), and risk of valve thrombosis (from 1.8 to 0.2 events per 100 patient-years). The benefits of VKA therapy after mechanical valve placement are marked.

The mainstay of treatment in both guidelines remains indefinite anticoagulation with a vitamin K antagonist (VKA). Mechanical Valve ReplacementĪfter valve repair with a mechanical device, the American College of Chest Physicians (ACCP) and the American College of Cardiology (ACC)/American Heart Association (AHA) 12,13 provide fairly similar recommendations regarding the use of anticoagulation (Tables 1 and 2). These considerations underscore the importance of addressing proper anticoagulation techniques to minimize postoperative thrombotic complications, while maintaining acceptable levels of risk related to bleeding. 10,11 Furthermore, the presence of atrial fibrillation, a common arrhythmia in patients with VHD (especially if involving the mitral valve), also requires lifelong anticoagulation in the majority of patients. 8,9 The risk of thromboembolic complications is greatest during the first three months after surgery for both mechanical and bioprosthetic devices, with persistent life-long risk for patients with mechanical valves. Thrombotic and embolic complications and anticoagulation-related bleeding are by far the most prevalent contributors to morbidity and mortality after surgery for VHD. 5,6 This may be attributable to the advancing technology in bioprosthetic device development, and the increasing rate of valve replacement surgery in the elderly, in whom bioprosthetic devices are preferred. 5,6 Observational data indicate a steady increase in the use of bioprosthetic valves, with a concurrent decrease in the use of mechanical prostheses. Surgical repair of VHD with either a mechanical or bioprosthetic valve is a common solution with ever-improving operative outcomes. 1-3 Current data estimate the overall prevalence of VHD in the United States to be 2.5%, with prevalence estimates in those over the age of 75 to be as high as 13.3%. Valvular heart disease (VHD) is a common contributor to cardiac morbidity and mortality.


 0 kommentar(er)
0 kommentar(er)
